With Hybrid Brains, These Mice Smell Like a Rat
If mice ever wonder what it’s like to experience the world as a rat, some are now able to live that dream, at least when it comes to the sense of smell.
Researchers led by Columbia’s Kristin Baldwin have created mice with hybrid brains—part mouse, part rat—that sense the odors of the world with their rat neurons.
It is the first time that an animal has been able to use the sensory apparatus of another to sense and respond accurately to the world and is one indication of how flexible the brain can be in integrating outside brain cells.
“This research is starting to show us how we can expand the flexibility of a brain so that it can accommodate other kinds of inputs, from human-machine interfaces or transplanted stem cells,” says Baldwin, professor of genetics & development at Columbia University Vagelos College of Physicians and Surgeons and in the Columbia Stem Cell Initiative.
Hybrid goals
One of the biggest challenges in understanding and treating diseases of the human brain is that it is impossible to fully understand these disorders with current research methods.
“We have beautiful models of cells in dishes and 3D cultures called organoids and they both have their advantages,” Baldwin says, “But none of them allow you to determine if the cells are really functioning at the highest level.”
Hybrid brains will allow researchers to better understand how brain cells get sick or die and better understand the rules of repairing and replacing parts of the brain.
“Right now, researchers are transplanting stem cells and neurons into people with Parkinson's and epilepsy. But we do not really understand how well that will work,” she adds. “With hybrid brain models, we can start to get some answers and at a faster pace than a clinical trial.”
Creating hybrid brains
Researchers have previously created hybrid brains by injecting neurons or transplanting pea-sized brain organoids from one species into either a developing brain or a fully formed one, either a mouse or rat.
“These experiments have told us that we are somewhat limited in when and how we can add brain cells to an existing brain,” Baldwin says. “If the brain has developed to a certain point, the transplanted cells don’t necessarily wire together appropriately.”
Instead, Baldwin’s team introduced rat stem cells into mouse blastocysts, an early stage in development that occurs just hours after fertilization, so that the rat and mouse cells could grow together and integrate on their own.
The technique, called blastocyst complementation, is similar to a technique used to create mice with human immune systems, which have proved to be powerful research tools. But until this study, the technique had not been successful in creating hybrid brains of two different species.
“What we’re doing is really cutting edge,” Baldwin says.
Rat neurons restore sense of smell in mice
In the team’s first hybrid experiments, researchers examined where rat neurons appeared in the mouse brain. Rats develop at a slower pace and have bigger brains, but in the mouse, the rat cells followed the mouse’s instructions, accelerating their development and making the same kinds of connections as their mouse counterparts.
“You could see rat cells throughout almost the entire mouse brain, which was fairly surprising to us,” Baldwin says. “It tells us that there are few barriers to insertion, suggesting that many kinds of mouse neurons can be replaced by a similar rat neuron.”
The researchers then looked to see if the rat neurons had been integrated in a functional neural circuit, in this case part of the olfactory system, which is essential to mice for finding food and avoiding predators. By engineering the mouse embryo to kill or inactivate its own olfactory neurons, the researchers could easily determine if rat neurons had restored the animals’ sense of smell.

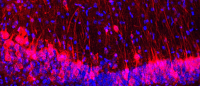
Mice that smell like a rat. In the hybrid brains, odor processsing centers (circular structures) are a mixture of rat and mouse neurons. Odor processing centers with red cells contain rat neurons; odor processing centers with green neurons contain mouse neurons. Image by Ben Throesch.
“We hid a cookie in each mouse cage, and we were very surprised to see that they could find it with the rat neurons," Baldwin says.
Some mice did better at finding the cookie than others, however. The researchers found that mice that retained their own silenced olfactory neurons were less successful at finding hidden cookies than mice whose olfactory neurons were engineered to disappear during development.
“This suggests that adding replacement neurons isn’t plug and play,” Baldwin says. “If you want a functional replacement, you may need to empty out dysfunctional neurons that are just sitting there, which could be the case in some neurodegenerative diseases and also in some neurodevelopmental disorders like autism and schizophrenia.”
With the hybrid brain system created by Baldwin’s team, researchers can now use the mice to carefully dissect what happened in the different models, which may eventually help improve the success of human cell transplantation.
Primate hybrids?
One downside of the new hybrid brain system is that the rat cells were randomly distributed in each different animal, a hurdle in extending these studies to other sensory and neural systems in the brain.
Baldwin’s lab is currently trying to find ways to drive the inserted cells to develop into just one cell type, which may allow for more precise experimentation.
If inserted cells can be constrained in their development within hybrid brains, it could also open the door to creating hybrid brains with primate neurons. “This would help us get even closer to understanding human disease,” Baldwin says.
References
More information
All authors: Benjamin T. Throesch (Scripps Research Institute and University of California, San Diego), Muhammad Khadeesh bin Imtiaz (University of Texas Southwestern Medical Center), Rodrigo Muñoz-Castañeda (Cold Spring Harbor Laboratory), Masahiro Sakurai (Salk Institute and UT-Southwestern), Andrea L. Hartzell (Scripps), Kiely N. James (Scripps and UCSD), Alberto R. Rodriguez (Scripps), Greg Martin (Scripps), Giordano Lippi (Scripps), Sergey Kupriyanov (Scripps), Zhuhao Wu (Icahn School of Medicine at Mount Sinai), Pavel Osten (Cold Spring Harbor), Juan Carlos Izpisua Belmonte (Salk Institute and Altos Labs), Jun Wu (Salk and UT-Southwestern), and Kristin K. Baldwin (Columbia, Scripps, and UCSD).
The research was supported by the National Institutes of Health (DP1DK113616, RF1AG079517, R56AG062618, DP1AG055944, R01MH102698, and R01DC012592), the Cancer Prevention & Research Institute of Texas (RR170076), Hamon Center for Regenerative Science & Medicine, Harold and Leila Y. Mathers Charitable Foundation, Moxie Foundation, Leona M. and Harry B. Helmsley Charitable Trust (2021-RG-MED002), Hewitt Foundation), Universidad Católica San Antonio de Murcia, Swiss National Science Foundation, and a fellowship from the American Epilepsy Society (5-79096).
Kristin Baldwin is on the scientific advisory board of Gameto Therapeutics. Other disclosures are noted in the article.
